Introduction
The purpose of this policy document is to set out the methods by which a repeat prescription will be issued and the roles and responsibilities within the practice.
There are Four Stages:
- Management control
- Initiation/ Request
- Production/ Authorisation
- Clinical control/ Review
The GP should retain an active involvement throughout the repeat prescribing process and should not delegate any entire part of the process to ancillary staff. Those stages in bold above are entirely the responsibility of the GP.
Initiation / Request
- The decision to transfer a drug from an acute prescription to a repeat prescription must always be made by the doctor after careful consideration of whether the drug has been effective, well-tolerated and is required long-term. (The patient should be seen, or at least spoken to, at this stage to ascertain this and check compliance). It is the duty of the doctor at this stage to ensure the patient understands the repeat prescribing process and what is required of them.
- Consideration should be given to alternative drugs and / or generic prescribing where appropriate.
- Care should be taken to ensure the repeat record is accurate, quantities for each drug are synchronised where possible and review dates are entered.
- Computerisation of repeat prescribing is to be encouraged and is the ultimate aim for all practices.
- Drugs should be linked by read code to medical conditions within the clinical system as appropriate.
Request
- This will largely be the responsibility of the patient.
- The patient should be given a list of drugs they are currently taking on repeat prescription, preferably as a computer-generated list (usually forming the right hand side of the prescription slip).
- The patient or his /her representative must have an active role in requesting a repeat prescription.
- The patient should be encouraged to indicate on the repeat request slip which drugs they require when a request is made. If they have left the form blank and it is not obvious from their computer record which medication is needed, then the patient should be contacted if possible, rather than all the medication given.
- Only urgent telephone requests or telephone requests from elderly and housebound patients can be taken. Patients should allow 48 hours for requests to be dealt with. This allows adequate time for a good quality repeat prescribing system to operate. For postal requests, to be returned via an SAE, patients should allow one week.
- Patients should be encouraged to tell their GP’s if they are no longer taking a repeat medication. The appropriateness of this can then be assessed and the computer updated to reflect the change.
- It is becoming more common for chemists to request repeat medication on behalf of patients. Whilst this has advantages it is worth bearing in mind that not all chemists check with the patient their monthly needs which can result in everything being ordered when it is not necessarily required. Spot checks with patients and chemists are advisable to ensure the correct dosage and issue of medication is being made to those patients.
Electronic Prescribing Service (EPS)
This is a new service that is intended to make it easier for GPs to issue prescriptions and more convenient for patients to collect their medicines.
Using EPS means that prescriptions by GPs and other prescribers will be transferred electronically to the pharmacist nominated by the patient. The prescriptions will also be sent automatically to the Prescriptions Pricing Authority (PPA).
A protocol for managing EPS within a practice could be as follows on the next page:

Production/ Authorisation
- This will usually be the responsibility of the receptionist/prescription clerk.
- Computer generated repeat prescriptions are good practice in that handwritten forms are prone to error.
- A compliance check is preferable at this stage and the computer should normally alert the user if medication appears to be over or under used. Particular attention should be paid to ‘as required’ drugs and if problems are suspected the doctor should be alerted, preferably before the prescription is produced.
- Practices should not supply further repeat prescriptions at shorter time intervals than have been authorised without agreeing the reason for the early request, e.g. holiday and documenting this reason in the patient’s medical record.
- Provided there appears to be no problem, a prescription can be generated and left for the doctor to authorise and sign, with the notes to hand (computerised or manual) as far as practically possible, to cross check the validity and appropriateness of the request. Situations where notes should always be available include:
- Where the request slip indicates that a review is necessary
- Where any drug requested by the patient is not on their repeat record
- Where any of the following drugs are requested:
- Temazepam
- Diazepam (Valium )
- DihyGPocodeine
- Paracetamol and codeine 500/30 preparations, e.g. Solpadol, Tylex
- PPI
- All controlled drugs
- Where the item requested has been issued less than one month previously.
- Any request about which the practice staff are concerned or uncertain.
- Where additions or corrections are made the doctor signing the prescription should initial or countersign against them. A record should be made of any subsequent handwritten alterations to computer-generated prescriptions.
- Blank prescriptions should never be signed by a doctor for later completion by
him/herself or a delegate. To do so is in breach of terms of service. - Unused space should be cancelled out under the last drug by a computerised mechanism or by the doctor deleting the space manually.
- All repeat prescriptions issued should be recorded on the computer.
- Practices should store prescriptions awaiting collection in a secure way and have a standard time limit for collection of repeat medication (e.g. four weeks) after which those not collected should be investigated, e.g. no longer required or medication underused etc., and then destroyed and noted in the patient’s medical record.
- It may be that patients need their medication to be placed in blister packs of 7 days. This is usually appropriate for elderly patients and those that have serious difficulties managing their medication. A request should be put in to the surgery by either the chemist, district nurse or support worker and this should be passes to a GP for approval. It is then usual to produce these prescriptions in 7 day dosages and 4 can be issued at any one time. Care must be given if a medication is switched part way through a prescription that the dossette boxes are also changed.
Clinical Control/ Review
- This is solely the responsibility of the doctor, although the nurse can review certain patients on behalf of the doctor, e.g.: contraception and asthma although patients may not necessarily have to be seen by the doctor. The review date is set on the computer for every 6 months. For those patients who need annual review, e.g. chronic stable conditions, reviewing them in their birthday month may serve to remind patients of their obligation to attend for review.
- A maximum of 28 days will be given for a prescription. A few patients could be given three month’s supply at GPs discretion e.g. when going on holiday or for certain types of medication – Oral contraceptives, HRT.
- When patients are on several regular long-term medications, quantities should be prescribed to synchronise repeat intervals. In the UK patient packs are moving towards multiples of 28 days (rather than 30)
- When patients are discharged from hospital, their regular medication may have changed. This is a particularly vulnerable time for errors to occur and ideally the doctor should amend the repeat record personally. A check of prescriptions not yet collected should also be made to ensure that it contains the correct medication.
The following considerations should be kept in mind by the doctor when carrying out medication review consultations:
- Control of the condition – is this optimal?
- Unnecessary medication – can anything be stopped?
- Compliance
- Is the patient taking the medication properly?
- Could the regimen be simplified?
- Is there a problem with unwanted adverse effects?
- Check understanding of medication?
- Monitoring – is this required, e.g. phenytoin levels, INR, TFTs, LFTs, U&Es
- Cost considerations – change to generics if appropriate, or consider change to a more cost-effective treatment (consider local formulary)
Management control
This would largely be the responsibility of the practice manager. Practice staff that write, or are involved in the preparation of, repeat prescriptions should be appropriately trained in the practice protocols for repeat prescribing, what their responsibilities are, and the need for accuracy. This should be on going, but is particularly important for new staff.
Liaison with local community pharmacists is essential if procedures are changed that may ultimately impact on them.
An adequate system for the secure storage and use of FP10s should be in place. A log sheet will be kept (& maintained by prescription clerk TB) for prescription pads coming into the practice (from PCSE) and distribution within the practice. This will be audited twice every year.
The practice computer system holding the prescribing records must be backed-up regularly.
Periodic audit of repeat prescribing will be carried out annually. This audit should also include prescription re-prints.
Setting up a repeat prescription:
The medication to be included on a repeat prescription should be agreed between GP and patient. Certain items should not be on repeat (& should be on acute only).
These include but are not limited to:
- Contraceptive pills
- HRT
- Salbutamol
- Controlled drugs
The importance of the need for regular review of repeat medication should be stressed to the patient.
It is the responsibility of the patient’s GP to ensure that an accurate up-to-date record of a patient’s repeat medication is held in their computer records and that all prescriptions are indicated / linked to a condition by read code.
Repeat medication prescriptions should last for an agreed length of time, usually 6-12 months, before medication should be reviewed (although this period can be extended if felt appropriate at the discretion of the prescribing GP).
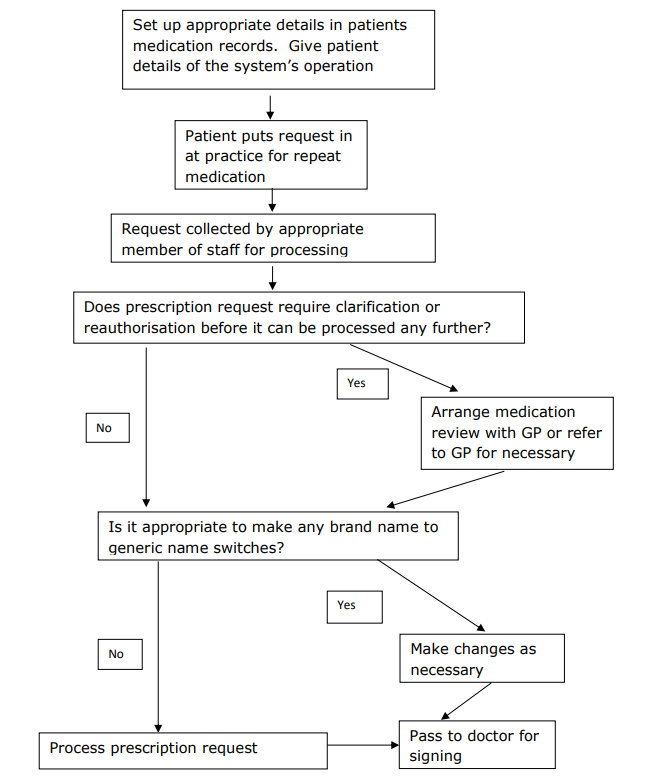
Provide patients with details of the system operation at an appropriate time (on registration with the practice, on commencing a repeat prescription). Posters detailing the operation of the system should be displayed around the practice.
Operation of the system
The practice staff are responsible for the day to day running of the system.
This should include:
- An appointed member of staff being given responsibility for the daily collection and processing of all repeat prescription requests.
- When preparing a repeat prescription, practice staff can make brand to generic name switches as appropriate (see attached list of those medications which should only be prescribed by brand name).
- Routine reauthorisation of repeat prescriptions is the responsibility of the Doctor. If items requested have expired and need reauthorisation the patient is required to attend a medication review, unless housebound. If housebound, the GP is then responsible for deciding whether to automatically re-authorise the repeat prescription or to provide a home visit.
When to refer the prescription back to the doctor
- If anything is unclear with a repeat prescription request refer back to the prescribing GP.
- If a patient requests an item which is not included or differs from the details recorded in their records, they should be referred to the GP.
- If a patient under or over orders items on their repeat prescription indicating poor compliance, this should be highlighted with the GP.
Monitoring of repeat prescribing
Ideally a GP should carry out a medication review when:
- A block of repeat medication comes to an end.
- Patients attend for monitoring of the condition requiring repeat
treatment. - Opportunistically should a patient attend with another complaint.
The review should consist of an assessment of the patient’s condition and compliance with prescribed medication. If any repeat medications are no longer being requested, an attempt should be made to ascertain the reason why and appropriate action taken.
Controlled Drugs
All controlled drugs not sent electronically (EPS) should be signed for by the person collecting the prescription e.g. patient / chemist.
Non-collection of prescriptions
Monthly checks will be carried out of prescriptions not collected (by the prescribing clerk). Prescriptions will be cancelled on the patient’s prescribing record & a note added. The prescribing clerk will task the GP to advise them that a prescription has not been collected & what details of the medication. The GP will then decide if any action needs to be taken i.e. follow up call / consultation if concerns (mental health patient etc.)
Repeat Prescribing Flowchart
Following agreement between patient and doctor to commence medication on a repeat prescription: